 Delivery Room resuscitation of VLBW infants can be initiated with less oxygen even with room air without concomitant overt morbidity. This change was associated with more infants with an initial PaO2 <80 mm Hg and lower saturation values on admission as well as a lower FiO2 requirement at 24 h.
Delivery Room resuscitation of VLBW infants can be initiated with less oxygen even with room air without concomitant overt morbidity. This change was associated with more infants with an initial PaO2 <80 mm Hg and lower saturation values on admission as well as a lower FiO2 requirement at 24 h.

































































PRETERM:
- extremely preterm (less than 28 weeks)
- very preterm (28 to 32 weeks)
- moderate to late preterm (32 to 37 weeks).
The problem
An estimated 15 million babies are born too early every year. That is more than 1 in 10 babies.
Many survivors face a lifetime of disability, including learning disabilities and visual and hearing problems.
Globally, prematurity is the leading cause of death in children under the age of 5 years.
The solution
More than three quarters of premature babies can be saved with feasible, cost-effective care, such as essential care during child birth and in the postnatal period for every mother and baby, provision of antenatal steroid injections (given to pregnant women at risk of preterm labour and under set criteria to strengthen the babies’ lungs), kangaroo mother care (the baby is carried by the mother with skin-to-skin contact and frequent breastfeeding) and antibiotics to treat newborn infections.
WHO recommendations on interventions to improve preterm birth outcomes
Delivery room handling of the newborn
Introduction
Delivery room handling of the newborn covers all procedures carried out on the newborn immediately following birth, including:
Heart rate assessment, suctioning, ventilation/sustained inflation,
provision of positive end-expiratory pressure (PEEP), cord clamping,
oxygen supplementation, and heat loss prevention.
This critical time period was first called “the golden minutes” by Vento et al. in 2009.
The following year, the International Liaison Committee on Resuscitation (ILCOR) emphasized the importance of the first minute of life using the term the Golden Minute.
Unfortunately, ILCOR did not define when the “Golden Minute” starts, and there has been a wide variation in practice as to when the clock should be started.
Therefore, we have previously emphasized the importance of a common definition with international agreement about when the Golden Minute begins.
A baby is born when the whole body is out, and that is when the clock is started and the Golden Minute begins.
During the first 30 s, the baby should be dried and kept warm.
For babies <28 weeks of gestational age (GA), this includes being wrapped in plastic without drying, and placement under a radiant warmer.
Neonates should be stimulated to breathe by rubbing the chest or the spine preferably in a caudocranial direction, which may lead to extension of the spine contributing to opening of the lungs.
The infant should also be positioned correctly to open the airway, and the heart rate and breathing rate should have been recorded within this time frame.
In the next 30 s, respiratory support should be established if needed and, if available, a pulse oximetry probe should be placed.
While these recommendations are important to optimize newborn outcomes, it might be unrealistic to reach all these goals in such a short period of time.
A recent study found that the median time to start auscultation of the heart is 62 s (inter-quartile range 40–79 s) and the first heart rate is available after 70 s.
Similarly, premature babies were placed in a plastic bag at 62 s (40–79) while pulse oximetry data were obtained at 78 s.


Newborn life support (NLS) is intended to provide this help and comprises the following elements:
- Enabling placental transfusion (when able to do so) by delaying the clamping of the umbilical cord.
- Drying and covering the newborn infant, and where necessary taking additional steps, to maintain a normal body temperature (i.e. between 36.5°C and 37.5°C).
- Assessing the infant’s condition and the need for any intervention.
- Maintaining an open airway.
- If the infant is not breathing, aerating the lungs with inflation breaths.
- Continue ventilating apnoeic infants until respiration is established.
- If the heart remains less than 60 min-1 after 5 effective inflation breaths and 30 seconds of effective ventilation, start chest compressions.
Term baby Vs Preterm Baby:
Prem vs term?
A baby born at term and a baby born preterm, will look and behave very differently. Let’s learn how.
A few definitions before we get started.
- The gestational age refers to the ‘age’ of the neonate during the pregnancy. It is measured in weeks.
- A term pregnancy refers to babies born after 37 weeks gestation.
- Babies born before 37 weeks are considered pre-term, more commonly known as premature.
- Babies born past 42 weeks are considered to be post-term.
That being said, there are stages of prematurity and post maturity. Let’s consider the World Health Organisation’s (2016) neonatal classifications in the table below, based on maturity at birth.
| Definition of maturity at birth | Completed weeks of gestation |
|---|---|
| Extremely preterm | < 28 |
| Very preterm | 28 – <32 |
| Moderate to late preterm | 32 – <37 |
| Term | 37 – 41 |
| Post-term | ≥42 weeks |
Consider the pictures below of the premature baby on the left, and the term baby on the right. What differences do you notice?
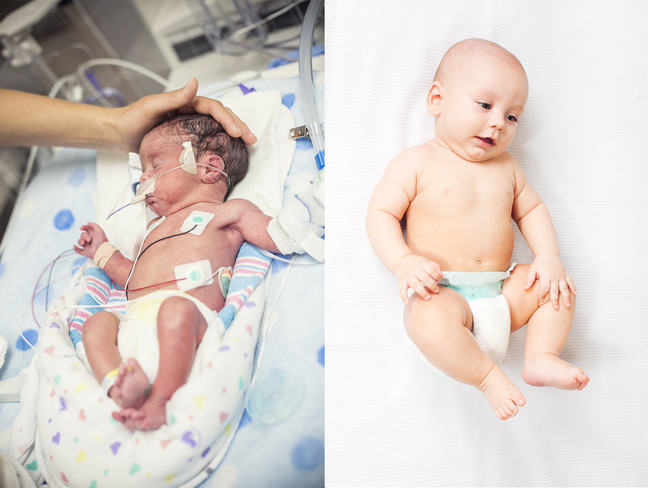
The most obvious difference is their size. Term babies have noticeably more body fat.
For example, you may notice breast tissue, chubby arms, legs and tummies. Premature babies appear ‘skinny’ in comparison.
Their lack of body fat – a type of fat known as brown fat that protects newborns from hypothermia, means they are at risk of becoming very cold, very quickly. This is one reason why it is so important to keep all babies, but especially premature babies warm.
Tone
The tone of the baby is another important difference. Term babies tend to adopt a frog-like, fully flexed posture. This posture also helps them to keep warm. Premature babies however, have a lack of body tone and are quite ‘floppy’ in comparison. This increases their risk of becoming cold after birth.
Skin colour
The skin colour difference between premature and term babies is also significant. Premature babies tend to look quite red. The more premature the baby, the more translucent and gelatinous their skin will look and feel. Premature skin is very fragile because the stratum corneum (the outermost layer of the skin) is underdeveloped (Oranges, Dini, & Romanelli, 2015).
Body hair
Did you know premature babies also tend to be quite hairy? This premature hair growth, lanugo,is the first hair produced by fetal hair follicles. Lanugo is present from around 20 weeks gestation. It is very fine, soft and usually not pigmented (Moore, Persaud, & Torchia, 2013). It can be found everywhere on a baby’s body, except on the palms, lips, and soles of the feet. Most fetuses develop lanugo around the fourth or fifth month of pregnancy and it disappears as the premature baby reaches term.
Other differences
In terms of behaviour, the body systems of the premature infant are also immature. This means that the baby is prone to
- apnoea – periods of 15 seconds or more, where they don’t breathe
- jaundice – due to immaturity of the liver
- feeding problems – because the suck/swallow reflex is not developed until 33 weeks gestation and
- delays in the transition of fetal to adult circulation (more on this later).
**************************************************************************
Delivery Room Resuscitation of Preterm Infants
A lung-protective strategy should start immediately after birth to establish a functional residual capacity, reduce volutrauma and atelectotrauma, facilitate gas exchange, and improve oxygenation during neonatal transition.
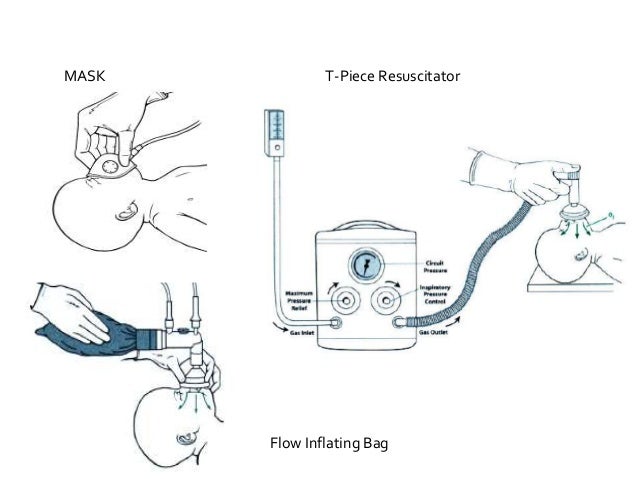
Interfaces During Respiratory Support in the DR
The Changing Landscape in Supporting Preterm Infants at Birth
Noninvasive ventilation for preterm infants at birth has been recommended and universally adopted. The umbilical cord is often clamped immediately in order to provide the support the infant needs for stabilization.
However, recent scientific data from experimental studies that involve animals in transition and human studies using physiological measurements at birth have increased awareness as to how little we know about how these interventions interact and integrate with the infant’s changing physiology.
It has become clear that in apneic infants the larynx is closed immediately after birth, which can completely negate the effect of noninvasive ventilation of the lung.
For this reason, stimulating and supporting spontaneous breathing could enhance the success of noninvasive ventilation.
Large swings in blood pressure, blood flow, and oxygenation caused by immediate cord clamping can be avoided by postponing cord clamping until lung aeration has been established.
Important guideline changes
The following are the changes that have been made to the NLS guidelines in 2015,some build on or expand the changes that were introduced in 2010.14,15
- For uncompromised term and preterm infants, a delay in cord clamping of at least one minute from the complete delivery of the infant, is now recommended. As yet there is insufficient evidence to recommend an appropriate time for clamping the cord in infants who are severely compromised at birth. For infants requiring resuscitation, resuscitative intervention remains the immediate priority. Stripping (or ‘milking’) of the cord is not recommended as a routine measure except in the context of further randomised trials.
- The temperature of newly born infants is actively maintained between 36.5°C and 37.5°C after birth unless a decision has been taken to start therapeutic hypothermia. The importance of achieving this has been highlighted and reinforced because of the strong association with mortality and morbidity. Even the mild hypothermia that was once felt to be inevitable and therefore clinically acceptable carries a risk. The admission temperature should be recorded as a predictor of outcomes as well as a quality indicator.
- Preterm infants of less than 32 weeks gestation may benefit from a combination of interventions to maintain their body temperature between 36.5°C and 37.5°C after delivery through stabilisation and neonatal unit admission. These may include;
- Warmed humidified respiratory gases16
- Thermal mattress alone17
- A combination of increased room temperature with plastic wrapping of head and body with thermal mattress18
All of these combinations have been effective in reducing hypothermia. In addition, the delivery room temperature should be at least 26°C for the most immature infants.
- An ECG, if available, can give a rapid accurate and continuous heart rate reading during newborn resuscitation.19 It does not, however, indicate the presence of a cardiac output and should not be the sole means of monitoring the infant.
- Resuscitation of term infants should commence in air. For preterm infants, a low concentration of oxygen (21–30%) should be used initially for resuscitation at birth. If, despite effective ventilation, oxygenation (ideally guided by oximetry) remains unacceptable, use of a higher concentration of oxygen should be considered. Blended oxygen and air should be given judiciously and its use guided by pulse oximetry. If a blend of oxygen and air is not available use what is available. If chest compressions are administered, supplemental oxygen should be increased.
- Attempts to aspirate meconium from the nose and mouth of the unborn infant, while the head is still on the perineum, are not recommended. The emphasis should be on initiating lung inflation within the first minute of life in non-breathing or ineffectively breathing infants and this should not be delayed. If presented with a floppy, apnoeic infant born through thick particulate meconium it is reasonable to inspect the oropharynx rapidly to remove potential obstructions. Tracheal intubation should not be routine in the presence of meconium and should only be performed for suspected tracheal obstruction.
- Nasal continuous positive airways pressure (CPAP) rather than routine intubation may be used to provide initial respiratory support of all spontaneously breathing preterm infants with respiratory distress. Early use of nasal CPAP should also be considered in those spontaneously breathing preterm infants who are at risk of developing respiratory distress syndrome (RDS).12,13,20
- The recommended compression: ventilation ratio for CPR remains at 3:1 for newborn resuscitation. Asynchronous compressions are not recommended.
Suggested sequence of actions
Keep the infant warm and assess
Infants are born small and wet. They get cold very easily, especially if they remain wet and in a draught. For uncompromised infants, a delay in cord clamping of at least one minute from the complete delivery of the infant, is recommended. Allowing placental transfusion ensures a more gradual transition to extra-uterine life preventing sudden changes in venous return to the heart and the potential impact of these on blood pressure.
Whatever the situation it is important that the infant does not get cold. In all cases whether intervention is required or not, dry the term or near-term infant, remove the wet towels, and cover the infant with dry towels. Significantly preterm infants are best placed, without drying, into polyethylene wrapping under a radiant heater. In infants of all gestations, the head should be covered with an appropriately sized hat. The temperature must be actively maintained between 36.5°C and 37.5°C after birth unless a decision has been taken to start therapeutic hypothermia. The admission temperature should always be recorded as a predictor of outcomes as well as a quality indicator.
This process will provide significant stimulation and will allow time to assess the infant’s breathing, and heart rate. A note should be made of the colour and tone, although these are of lesser importance in determining the immediate approach to be taken they can point towards the severely acidaemic baby (potentially requiring substantial resuscitation) or anaemic baby (potentially requiring urgent transfusion).
Reassess breathing and heart rate regularly every 30 s or so throughout the resuscitation process but it is the heart rate, which is the key observation. The first sign of any improvement in the infant will be an increase in heart rate. Consider the need for help; if needed, ask for help immediately.
A healthy infant will be born blue but will have good tone, will cry within a few seconds of delivery and will have a good heart rate within a few minutes of birth (the heart rate of a healthy newborn infant is about 120–150 min-1). A less healthy infant will be blue at birth, will have less good tone, may have a slow heart rate (less than 100 min-1), and may not establish adequate breathing by 90–120 s. An unwell infant will be born pale and floppy, not breathing and with a slow, very slow or undetectable heart rate.
In the first few minutes, the heart rate of an infant is usually judged best by listening with a stethoscope. It may also be felt by gently palpating the umbilical cord but a slow or absent rate at the base of the umbilical cord is not always indicative of a truly slow heart rate. Feeling for any of the peripheral pulses is not helpful.
An ECG is the most accurate way to obtain a rapid and continuous heart rate reading but may not be immediately available, nor will it give an indication of a cardiac output.19 A pulse oximeter can give a continuous heart rate and oximetry reading in the delivery room. With practice it is possible to attach a pulse oximeter probe and to obtain a useful reading of heart rate and oxygen saturation about 90 s after delivery.21
Figure 1. Newborn life support algorithm
Airway
Before the infant can breathe effectively the airway must be open. The best way to achieve this is to place the infant on his back with the head in the neutral position (i.e. with the neck neither flexed nor extended). Most newborn infants will have a relatively prominent occiput, which will tend to flex the neck if the infant is placed on his back on a flat surface. This can be avoided by placing some support under the shoulders of the infant, but take care not to overextend the neck. If the infant is very floppy (i.e. has no or very little tone) it is usually necessary to support the jaw with a jaw thrust. These manoeuvres will be effective for the majority of infants requiring airway stabilisation at birth.
Airway suction immediately following birth should be reserved for infants who have obvious airway obstruction that cannot be rectified by appropriate positioning and in whom material is seen in the airway. Rarely, material (e.g. mucus, blood, meconium, vernix) may be blocking the oropharynx or trachea. In these situations, direct visualisation and suction of the oropharynx should be performed. For tracheal obstruction, intubation and suction during withdrawal of the endotracheal tube may be effective. This latter manoeuvre should only be performed by appropriately trained staff and, if performed, should not unduly delay the onset of inflation breaths and subsequent ventilation.
Breathing
Most infants have a good heart rate after birth and establish breathing by about 90 s. If the infant is not breathing adequately aerate the lungs by giving 5 inflation breaths, preferably using air.
Until now the infant’s lungs will have been filled with fluid. Aeration of the lungs in these circumstances is likely to require sustained application of pressures of about 30 cm H2O for 2–3 s; these are ‘inflation breaths’. Begin with lower pressures (20–25 cm H2O) in preterm infants.
Use positive end-expiratory pressure (PEEP) of 4–5 cm H2O if possible. It is of demonstrable benefit in preterm infants but should also be used in term infants, although evidence for its benefit in this group of infants is lacking from human studies.
If the heart rate was below 100 min-1 initially then it should rapidly increase as oxygenated blood reaches the heart.
- If the heart rate does increase then you can assume that you have successfully aerated the lungs.
- If the heart rate increases but the infant does not start breathing for himself, then continue ventilations at a rate of about 30–40 min-1 until the infant starts to breathe on his own.
- If the heart rate does not increase following inflation breaths, then either you have not aerated the lungs or the infant needs more than lung aeration alone. By far the most likely is that you have failed to aerate the lungs effectively. If the heart rate does not increase, and the chest does not passively move with each inflation breath, then you have not aerated the lungs.
If the lungs have not been aerated then consider:
- Checking again that the infant’s head is in the neutral position?
- Is there a problem with face mask leak?
- Do you need jaw thrust or a two-person approach to mask inflation?
- Do you need a longer inflation time? – were the inspiratory phases of your inflation breaths really of 2–3 s duration?
- Is there an obstruction in the oropharynx (laryngoscope and suction)?
- Will an oropharyngeal (Guedel) airway assist?
- Is there a tracheal obstruction?
Check that the infant’s head and neck are in the neutral position; that your inflation breaths are at the correct pressure and applied for sufficient time (2–3 s inspiration); and that the chest moves with each breath.
If the chest still does not move, ask for help in maintaining the airway and consider an obstruction in the oropharynx or trachea, which may be removable by suction under direct vision. An oropharyngeal airway may be helpful.
If the heart remains slow (less than 60 min-1) or absent after 5 effective inflation breaths and 30 seconds of effective ventilation, start chest compressions.
If you are dealing with a preterm infant then initial CPAP of approximately 5 cm H2O, either via a face mask or via a CPAP machine, is an acceptable form of support in infants who are breathing but who show signs of, or are at risk of developing, respiratory distress.
In preterm infants who do not breathe or breathe inadequately, you should use PEEP with your inflation breaths and ventilations as the lungs in these infants are more likely to collapse again at the end of a breath; using PEEP prevents this.
Chest compression
Almost all infants needing help at birth will respond to successful lung inflation with an increase in heart rate within 30 seconds followed quickly by normal breathing.11,22-24 However, in some cases chest compression is necessary. Chest compression should be started only when you are sure that the lungs have been aerated successfully.
In infants, the most efficient method of delivering chest compression is to grip the chest in both hands in such a way that the two thumbs can press on the lower third of the sternum, just below an imaginary line joining the nipples, with the fingers over the spine at the back. Compress the chest quickly and firmly, reducing the antero-posterior diameter of the chest by about one third.25
The ratio of compressions to inflations in newborn resuscitation is 3:1.
Chest compressions move oxygenated blood from the lungs back to the heart. Allow enough time during the relaxation phase of each compression cycle for the heart to refill with blood. Ensure that the chest is inflating with each breath. You should increase the oxygen concentration if you have reached this stage of resuscitation. You should also have called for help and a pulse oximeter, if not already in use, will be helpful in monitoring how you are doing.
Do not use asynchronous compressions, even if the infant has a tracheal tube placed, as maintaining air entry into the lung remains as important now as it was during the initial aeration. Compressing the chest during a ventilation breath may reduce air entry, which may be harmful.
In a very few infants (less than one in every thousand births) inflation of the lungs and effective chest compression will not be sufficient to produce an effective circulation. In these circumstances drugs may be helpful.
Drugs
Drugs are needed rarely and only if there is no significant cardiac output despite effective lung inflation and chest compression. The outlook for most infants at this stage is poor although a small number have had good outcomes after a return of spontaneous circulation followed by therapeutic hypothermia.
The drugs used include adrenaline (1:10,000), occasionally sodium bicarbonate (ideally 4.2%), and glucose (10%). All resuscitation drugs are best delivered via an umbilical venous catheter or if this is not possible through an intraosseous needle.26,27
The recommended intravenous dose for adrenaline is 10 microgram kg-1 (0.1 mL kg-1 of 1:10,000 solution). If this is not effective, a dose of up to 30 microgram kg-1 (0.3 mL kg-1 of 1:10,000 solution) may be tried.
Adrenaline is the only drug that may be given by the tracheal route, although of unknown efficacy at birth. If this is used, it must not interfere with ventilation or delay acquisition of intravenous access. The tracheal dose is thought to be between 50–100 microgram kg-1.
Use of sodium bicarbonate is not recommended during brief resuscitation. If it is used during prolonged arrests unresponsive to other therapy, it should be given only after adequate ventilation and circulation (with chest compressions) is established. The dose for sodium bicarbonate is between 1 and 2 mmol of bicarbonate kg-1 (2–4 mL kg-1 of 4.2% bicarbonate solution).
The dose for glucose (10%) is 2.5 mL kg-1 (250 mg kg-1) and should be considered if there has been no response to other drugs delivered through a central venous catheter.
Very rarely, the heart rate cannot increase because the infant has lost significant blood volume. If this is the case, there is often a clear history of blood loss from the infant, but not always. Use of isotonic crystalloid rather than albumin is preferred for emergency volume replacement. In the presence of hypovolaemia, a bolus of 10 mL kg-1 of 0.9% sodium chloride or similar given over 10–20 s will often produce a rapid response and can be repeated safely if needed.
When to stop resuscitation
In a newly-born infant with no detectable cardiac activity, and with cardiac activity that remains undetectable for 10 min, it is appropriate to consider stopping resuscitation. The decision to continue resuscitation efforts beyond 10 min with no cardiac activity is often complex and may be influenced by issues such as the availability of therapeutic hypothermia and intensive care facilities, the presumed aetiology of the arrest, the gestation of the infant, the presence or absence of complications, and the parents’ previous expressed feelings about acceptable risk of morbidity.
The difficulty of this decision-making emphasises the need for senior help to be sought as soon as possible.
Where a heart rate has persisted at less than 60 min-1 without improvement, during 10–15 min of continuous resuscitation, the decision to stop is much less clear.
No evidence is available to recommend a universal approach beyond evaluation of the situation on a case-by-case basis by the resuscitating team and senior clinicians.
Communication with the parents
It is important that the team caring for the newborn baby informs the parents of the baby’s progress. At delivery, adhere to the routine local plan and, if possible, hand the baby to the mother at the earliest opportunity. If resuscitation is required inform the parents of the procedures undertaken and why they were required. Record all discussions and decisions in the baby’s records as soon as possible after birth.
6. Post-resuscitation care
Therapeutic hypothermia
Term or near-term infants, with evolving moderate to severe hypoxic-ischaemic encephalopathy, should be treated with therapeutic hypothermia.28-31 Whole body cooling and selective head cooling are both appropriate strategies.28-33 Cooling should be initiated and conducted under clearly-defined protocols with treatment in neonatal intensive care facilities and the capabilities for multidisciplinary care. Treatment should be consistent with the protocols used in the randomised clinical trials (i.e. commence cooling within 6 h of birth, continue the cooling for 72 h before re-warming the infant over a period of at least 4 h). All treated infants should be followed longitudinally. Passive or active therapeutic hypothermia should only instituted following a senior clinical decision.
Glucose
Infants who are preterm or require significant resuscitation should be monitored and treated to maintain blood glucose in the normal range. Hypoglycaemia should be avoided in babies demonstrating signs of evolving hypoxic ischaemic encephalopathy or who are undergoing therapeutic hypothermia. An infusion of 10% glucose rather than repeated boluses is usually best at treating low blood glucose values and maintaining glucose in the normal range.
7. Explanatory Notes
Resuscitation or stabilisation
Most infants born at term need no resuscitation and they can usually stabilise themselves during the transition from placental to pulmonary respiration very effectively.
Provided attention is paid to preventing heat loss (and avoiding over-warming) and a little patience is exhibited before cutting the umbilical cord, intervention is rarely necessary.
However, some infants will have suffered stresses or insults during labour. Help may then be required which is characterised by interventions designed to rescue a sick or very sick infant and this process can then reasonably be called resuscitation.
Significantly preterm infants, particularly those born below 30 weeks gestation, are treated differently. Most infants in this group are healthy at the time of delivery and yet all can be expected to benefit from help in making the transition.
Maintaining the temperature between 36.5°C and 37.5°C is even more important than for term babies. Intervention in this situation is usually limited to keeping an infant healthy during this transition and is more appropriately called stabilisation.
Gentle airway support using CPAP rather than ventilation may be adequate for many of these infants. In the past both situations have been referred to as resuscitation and this seems inappropriate and likely to cause confusion.
Umbilical cord clamping
For healthy term infants delaying cord clamping for at least one minute or until the cord stops pulsating following delivery improves iron status through early infancy.34
For preterm infants in good condition at delivery, delaying cord clamping for up to 3 min results in increased blood pressure during stabilisation, a lower incidence of intraventricular haemorrhage and fewer blood transfusions.
However, infants were more likely to receive phototherapy. There are limited data on the hazards or benefits of delayed cord clamping in the non-vigorous infant.
Delaying cord clamping for at least one minute is recommended for all newborn infants not requiring resuscitation.
At present there is insufficient evidence to define an appropriate time to clamp the cord in infants who apparently need resuscitation.
However, this may be because time is the wrong defining parameter and perhaps the cord should not be clamped until the infant has started breathing (or the lungs are aerated).
Stripping (or ‘milking’) of the umbilical cord has been suggested as an alternative to delayed cord clamping when the infant is in need of resuscitation; however there is insufficient evidence to recommend this as a routine measure.
Umbilical cord milking did, however, produce improved short term haematological outcomes, admission temperature and urine output when compared to delayed cord clamping in once recent study.
Maintaining normal temperature (between 36.5°C and 37.5°C)
Naked, wet, newborn infants cannot maintain their body temperature in a room that feels comfortably warm for adults. Infants who are compromised are particularly vulnerable to the effects of cold stress, which may will lower arterial oxygen tension and increase metabolic acidosis.
Active measures will need to be taken to avoid hypothermia, especially in the preterm infant where a team approach and a combination of strategies may be required.
The neonatal unit admission temperature of newborn infants is a strong predictor of mortality at all gestations and in all settings.
For each 1°C decrease in admission temperature below this range there is an associated increase in mortality by 28%.
Babies born outside the normal delivery environment may benefit from placement in a food grade polyethylene bag or wrap after drying and then swaddling.
Alternatively, well newborns >30 weeks gestation who are breathing may be dried and nursed with skin to skin contact or kangaroo mother care to maintain their temperature whilst they are transferred. They should be protected from draughts.
Recommendation
Unless you have decided to implement therapeutic hypothermia, take active steps to maintain the temperature of the newly born infant between 36.5°C and 37.5°C from birth to admission and throughout stabilisation.
If the resuscitation is prolonged, consider measuring temperature during the resuscitation.
Oximetry and the use of supplemental oxygen
If resources are available, use pulse oximetry for all deliveries where it is anticipated that the infant may have problems with transition or need resuscitation. Oxygen saturation and heart rate can be measured reliably during the first minutes of life with a modern pulse oximeter. Data from healthy spontaneously breathing infants has been used to inform when oxygen should be given (see algorithm).55
The sensor must be placed on the right hand or wrist to obtain an accurate reading of the preductal saturation.56,57 Placement of the sensor on the infant before connecting to the instrument may result in faster acquisition of signal. In most cases a reliable reading can be obtained within 90 s of birth.58 Pulse oximetry can also provide an accurate display of heart rate during periods of good perfusion.
In healthy term infants, oxygen saturation increases gradually from approximately 60% soon after birth to over 90% at 10 min. In preterm infants hyperoxaemia is particularly damaging and if oxygen is being used and the saturation is above 95% the risk of hyperoxaemia is high. Therefore the rate of rise in oxygen saturation after birth in preterm infants should not exceed that seen in term infants, although some supplemental oxygen may be required to achieve this.57,59
Colour
Using colour as a proxy for oxygen saturation is usually inaccurate.60 However, noting whether an infant is initially very pale and, therefore, either acidotic or anaemic at delivery may be useful as an indicator for later therapeutic intervention.
ECG monitoring of heart rate
Clinical assessment of heart rate, whether by palpation of the cord or apex of the heart or by listening with a stethoscope tends to be inaccurate.61,62 There is increasing evidence supporting the use of ECG monitoring as a means of rapidly determining the heart rate during resuscitation; it is quicker to provide an accurate reading than pulse oximetry but does require that the ECG leads make good contact with the skin.19,63
Airway suctioning with or without meconium
Routine elective intubation and suctioning of vigorous infants at birth, does not reduce meconium aspiration syndrome (MAS).64 Nor does suctioning the nose and mouth of such infants on the perineum and before delivery of the shoulders (intrapartum suctioning).65 Even in non-vigorous infants born through meconium-stained amniotic fluid who are at increased risk of MAS, intubation and tracheal suctioning has not been shown to improve the outcome.66-68 There is no evidence to support suctioning of the mouth and nose of infants born through clear amniotic fluid.
Recommendation
Routine intrapartum oropharyngeal and nasopharyngeal suctioning for infants born with clear and/or meconium-stained amniotic fluid is not recommended.
The practice of routinely performing direct oropharyngeal and tracheal suctioning of non-vigorous infants after birth with meconium-stained amniotic fluid was based upon poor evidence. The presence of thick, viscous meconium in a non-vigorous infant is the only indication for initially considering visualising the oropharynx and suctioning material, which might obstruct the airway. If an infant born through meconium-stained amniotic fluid is also floppy and makes no immediate respiratory effort, then it is reasonable to rapidly inspect the oropharynx with a view to removing any particulate matter that might obstruct the airway. Tracheal intubation should not be routine in the presence of meconium and is performed only for suspected tracheal obstruction.68-72 The emphasis is on initiating ventilation within the first minute of life in non-breathing or ineffectively breathing infants and this should not be delayed, especially in the bradycardic infant.
Laryngeal mask
Several studies have shown that laryngeal mask airways (LMAs) can be used effectively at birth to ventilate the lungs of infants weighing over 2000 g, greater than 33 weeks gestation and apparently needing resuscitation.73-77 Case reports suggest that LMAs have been used successfully when intubation has been tried and failed – and occasionally vice-versa. One small randomised study has suggested that LMAs may reduce the need for intubation compared to facemask ventilation,78 however LMAs cost about three times as much and it is not clear how many of the infants resuscitated using an LMA would have responded to good quality facemask ventilation. Data on LMA use in smaller or less mature infants are scarce.
Recommendation
Consider using an LMA during resuscitation of the newborn infant if face mask ventilation is unsuccessful and tracheal intubation is unsuccessful or not feasible. The LMA may be considered as an alternative to a face mask for positive pressure ventilation among newborn infants weighing more than 2000 g or delivered ≥34 weeks gestation.78 The LMA may be considered as an alternative to tracheal intubation as a secondary airway for resuscitation among newborn infants weighing more than 2000 g or delivered ≥34 weeks gestation.78 There is limited evidence evaluating its use for newborn infants weighing <2000 g or delivered <34 weeks gestation and none for those infants receiving compressions.
Use of the LMA, nonetheless, should be limited to those individuals who have been trained to use it. Its use has not been evaluated in the setting of meconium stained fluid, during chest compressions, or for the administration of emergency intra-tracheal medications.
Exhaled carbon dioxide
Detection of exhaled carbon dioxide confirms tracheal intubation in neonates with a cardiac output more rapidly and more accurately than clinical assessment alone. It will not, however, distinguish between correct placement with the tip of the tracheal tube in the trachea and incorrect insertion with the tip in the right main bronchus (i.e. too long). False negative readings may occur in very low birth weight neonates and in infants during cardiac arrest (in these cases a brief period of chest compressions may bring about a colour change as more carbon dioxide is delivered to the lungs). False positives may occur with colorimetric devices contaminated with adrenaline, surfactant and atropine.
Drugs in resuscitation at birth
Ventilation and chest compression may fail to resuscitate fewer than 1 in 1000 infants.23 In this group, resuscitation drugs may be justified. Whilst there is evidence from animal studies for both adrenaline and sodium bicarbonate in bringing about return of spontaneous circulation, there is no placebo-controlled evidence in human infants for the effectiveness of any drug intervention in this situation. Even for adults and children in cardiac arrest, there is insufficient evidence to suggest that vasopressors improve long-term survival.
For this reason use of drugs before achieving lung aeration followed by chest compressions (known to be effective resuscitative interventions) can never be justified.12,13
Glucose
Hypoglycaemia is associated with adverse neurological outcome in a neonatal animal model of hypoxia and resuscitation.79 Newborn animals that were hypoglycaemic at the time of a hypoxic-ischemic insult had larger areas of cerebral infarction and/or decreased survival compared to controls.80,81 However, only a single clinical study has shown an association between hypoglycaemia and poor neurological outcome following perinatal hypoxia.82 In adults, children and extremely low-birth-weight infants receiving intensive care, hyperglycaemia is associated with a worse outcome.80-84 However, in children, hyperglycaemia after hypoxia-ischaemia does not appear to be harmful,85 which confirms data from animal studies86 some of which suggest it may even be protective.87 The situation remains unclear and unfortunately, the range of blood glucose concentration that is associated with the least brain injury following asphyxia and resuscitation cannot be defined based on available evidence.
*************************************************************************************










